When a drug’s patent runs out, prices don’t just dip-they plummet. It’s not a slow fade. It’s a crash. And it’s not just about one company losing money. It’s about millions of patients paying less, insurers spending less, and entire healthcare systems breathing easier. The moment a patent expires, the game changes. And the winner? The patient.
What Happens the Day After a Patent Expires?
The day after a patent expires, generic drug makers can legally sell the exact same medicine at a fraction of the cost. No new research. No new clinical trials. Just the same active ingredient, same dosage, same effect. But because dozens of companies can now make it, competition kicks in-and fast. The first generic usually drops the price by 15% to 20%. Sounds modest? Wait. By the time the third or fourth generic hits the market, prices often fall by 50%. By year five, with 10 or more generic makers competing, the drug can cost 80% less than the brand-name version. In the U.S., some drugs that once cost $800 a month now cost $10. That’s not a discount. That’s a revolution. Take Eliquis (apixaban), a blood thinner. Before its patent expired in 2020, patients paid up to $850 a month. After generics arrived, the same pill cost $10. Same pill. Same effectiveness. Just no patent protection.Why Do Prices Drop So Hard?
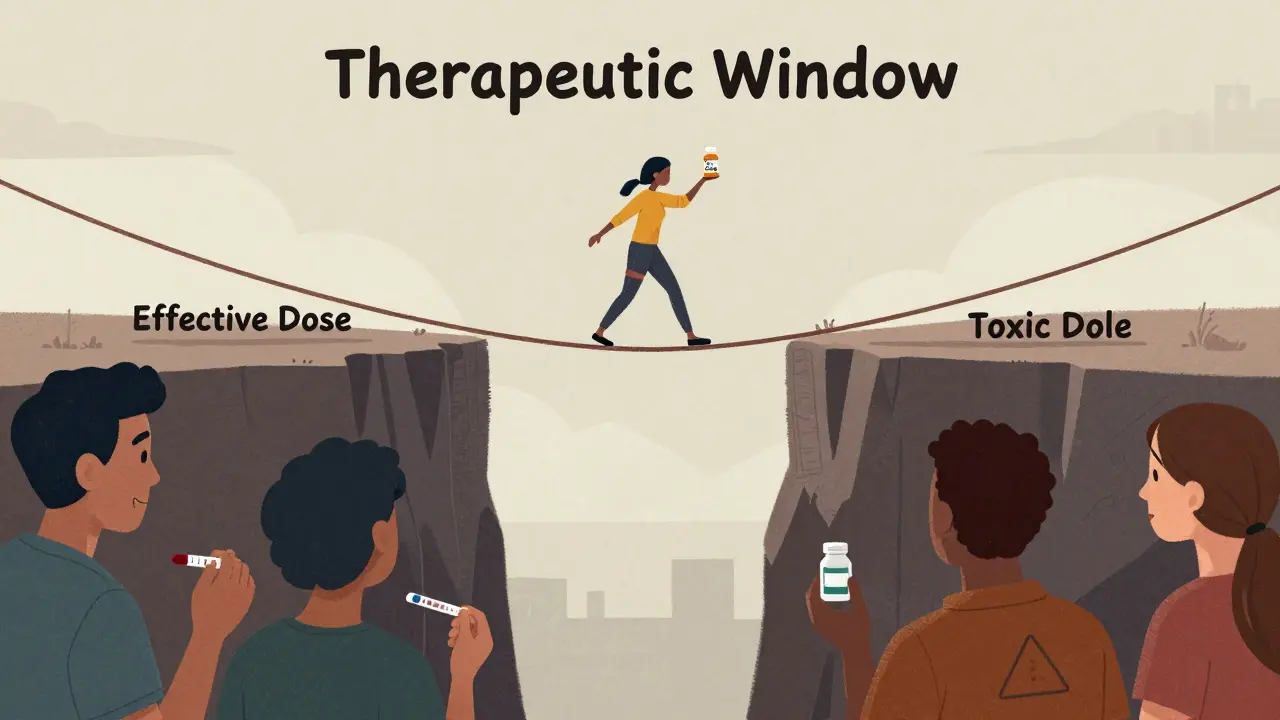
It’s basic economics: monopoly vs. competition. When one company owns a patent, they’re the only game in town. They can charge whatever they want. No one else can make it. That’s why drugs like Humira cost $7,000 a month in the U.S. before generics. But once the patent expires, it’s open season. Generic manufacturers don’t need to spend hundreds of millions on R&D. They just need to prove their version works the same. That process is cheaper, faster, and simpler. So they can undercut the brand by 80%, 90%, even 95%. And they do. Because if one generic company charges $100, another will charge $80. Then $60. Then $30. And so on. The race to the bottom isn’t cruel-it’s necessary. It’s how markets fix broken pricing.Not All Drugs Are Created Equal
Small molecule drugs-like pills for high blood pressure, diabetes, or cholesterol-see the fastest and deepest price drops. Their formulas are simple to copy. The FDA approves generics for these in about 10 months. But biologics? Those are different. These are complex drugs made from living cells-like Humira, Enbrel, or Ozempic. They’re not pills. They’re proteins. Copying them isn’t like copying aspirin. That’s why we call their copies “biosimilars,” not generics. Biosimilars take years longer to get approved. They need expensive testing. And originator companies fight hard to delay them. AbbVie, the maker of Humira, filed over 130 secondary patents on minor changes-like packaging or dosing-to stretch exclusivity. Even after the main patent expired in 2016, Humira stayed near full price until 2023, when Amgen’s Amjevita finally launched. And even then, prices didn’t drop overnight. Insurance companies and pharmacy benefit managers (PBMs) still used rebates and formulary rules to keep Humira on top. Patients didn’t see savings until they switched plans-or until states passed laws forcing substitution.
Why Do Some Countries See Smaller Price Drops?
The U.S. isn’t the only country with this problem. But it’s the worst. In 2023, a global study of 505 drugs showed that after eight years, prices fell:- 82% in the U.S.
- 60% in the UK
- 58% in Germany
- 48% in Canada
- 18% in Switzerland
The Hidden Barriers: Patents Within Patents
Here’s the ugly truth: many drugs never truly lose protection. Companies file “secondary patents” on tiny changes-new tablet coatings, different dosing schedules, even new packaging. These don’t make the drug better. They just delay competition. A 2025 report found that 78% of new patents filed for top-selling drugs weren’t for new medicines. They were for old ones. And 70% of the 100 most-prescribed drugs had their exclusivity extended at least once. Some, like semaglutide (Ozempic, Wegovy), have over 140 patents stacked on top of each other. The original patent expires in 2026. But with all the secondary ones? The drug could stay monopoly-priced until 2036. This isn’t innovation. It’s legal gaming. And it costs patients billions. The I-MAK group estimates that these patent thickets delay affordable access by an average of 4.2 years per drug.






9 Comments
The moment a patent expires, it’s not just a price drop-it’s a systemic reckoning. Generic manufacturers don’t need R&D, clinical trials, or marketing budgets-they just need a lab and a FDA form. And suddenly, a $800/month drug becomes $10. That’s not capitalism. That’s justice. 🚨💊
But here’s the kicker: PBMs and insurers still collude to keep brand-name drugs on formularies because they get kickbacks. You think this is about patient care? Nah. It’s about rebates. And until Congress cracks down on those, patients will keep paying more than they should. 😤
I’ve seen this firsthand-my dad switched from Eliquis to apixaban generics after his insurance finally updated. He was scared at first, but his doctor reassured him. The bloodwork didn’t change. His kidney function stayed stable. He’s been saving $700 a month ever since.
It’s wild how something so simple-copying a molecule-can be blocked by legal loopholes. I wish more people knew they could ask for generics. Even if their plan doesn’t list it, cash price is often cheaper than the copay. Just… ask. 🤍
Biologics are the real nightmare. Humira took seven years to get a biosimilar. Seven years. And even then, most pharmacies don’t stock them because the reps push the brand harder. It’s not about efficacy-it’s about distribution networks and pharmacy contracts. The system is rigged. No wonder people think generics are ‘inferior.’ They’ve never even been given the chance.
Also, doctors need better education. Too many still write ‘DAW’ without knowing what it means.
usa always overpay for everything lol
in india we get generics for 10 rupees even before patent expires
why? because we dont have billion dollar pharma lobbies
you people pay for their yachts and mansions
its sad
also why do you need 140 patents for one pill
its not a spaceship its a molecule
so dumb
Oh wow America finally figured out capitalism? 🙃
Meanwhile in India we’ve been getting $0.50 diabetes meds since 2008. You think patents are for innovation? Nah. They’re for corporate extortion. And you call yourselves a free market? Bro. Your system is a meme.
Also, Ozempic with 140 patents? That’s not science. That’s a legal heist. 😂💸
Let us not forget: the pharmaceutical industry is not a benevolent entity-it is a profit-maximizing machine, embedded within a regulatory framework that has, for decades, been captured by lobbying interests that prioritize shareholder returns over human life.
And yet, the narrative persists: ‘patents incentivize innovation.’ But when 78% of patents are secondary, trivial, and legally manipulative-when the original molecule remains unchanged for 30 years-it is not innovation that is being rewarded. It is rent-seeking. It is economic parasitism.
And the most tragic irony? The patients who suffer most are not the uninsured-they are the insured, who believe they are protected, while their PBMs quietly accept kickbacks from the very companies that price-gouge them.
And still, we are told to ‘be patient.’ Patient? We are the patients. And we are dying while the system profits.
…and yet, somehow, we are the ones who must ‘ask our doctors.’
Hey everyone-this is huge. If you’re on a brand-name drug right now, don’t wait for your insurance to catch up. Go to GoodRx. Check the cash price. I’ve seen people pay $12 for a 30-day supply of metformin when their copay was $45.
And if your doctor says ‘dispense as written’? Ask why. Is it because the generic doesn’t work? Or because they got a free lunch from the rep? Don’t be afraid to push back.
Also-ask your pharmacist if they have the generic in stock. Most do. They just don’t always tell you.
You have power. Use it. You’re not helpless. 💪
The real story here isn’t just about price drops-it’s about systemic inertia. Even when generics are available, the infrastructure to deliver them is broken. Pharmacies don’t have automated substitution systems. Pharmacists aren’t trained to advocate. Prescribers don’t know the rules. Insurers don’t update formularies for months. Patients don’t know to ask.
And it’s not just about cost-it’s about trust. People think generics are ‘weaker’ because they’ve never seen the data. The FDA requires bioequivalence within 80-125% of the brand. That’s not a guess. That’s science.
But until we fix the communication gap-the education gap-the policy gap-we’re just shouting into a void. And the people who lose? The ones who can’t afford to wait.
It’s not just about patents. It’s about how we’ve designed a system that assumes patients are too dumb to understand their own care.
And that’s the real tragedy.
Let’s be brutally honest: the 80% price drop narrative is misleading. It ignores the fact that most patients never pay the list price. Insurers negotiate rebates. PBMs take a cut. The ‘$850 Eliquis’ is a sticker price-a fiction. The actual net price was always lower.
And now? Generics have even lower net prices. But the savings aren’t flowing to patients-they’re flowing to PBMs and insurers. You think you’re saving $800? You’re saving $15. The rest is absorbed by middlemen.
Also, biosimilars aren’t cheaper because they’re better-they’re cheaper because they’re not patented. But they still cost $10k/year. So the ‘revolution’ is only revolutionary if you’re comparing list prices to cash prices. For most, it’s just a different kind of grind.
And don’t get me started on how the FDA’s accelerated approval process is just a way to offload risk onto patients.
This isn’t a win. It’s a redistribution of exploitation.