Key Takeaways
- Central diabetes insipidus (CDI) is caused by a shortage of antidiuretic hormone (ADH) from the posterior pituitary.
- Pituitary gland dysfunction can directly impair ADH release, creating a clear physiological link.
- Typical symptoms-excessive urine output, intense thirst, and electrolyte shifts-often overlap, making diagnosis tricky.
- Water‑deprivation testing, serum sodium measurement, and high‑resolution MRI are the core diagnostics.
- Desmopressin replacement restores water balance, but managing underlying pituitary disease may require surgery, hormone replacement, or radiotherapy.
Ever wondered why some patients with pituitary problems end up drinking gallons of water every day? The answer lies in how the brain’s water‑control system works. When the pituitary gland can’t release enough antidiuretic hormone, the kidneys can’t re‑absorb water, and a condition called central diabetes insipidus emerges. This article untangles the connection, explains the biology, and gives clear steps for clinicians and patients to navigate the overlap.
What Is Central Diabetes Insipidus?
Central Diabetes Insipidus is a rare disorder where the brain fails to produce enough antidiuretic hormone (ADH), also known as vasopressin, leading to the excretion of large volumes of dilute urine. The term “diabetes” refers to the excess urine, not to blood‑sugar problems. Most cases arise from damage to the hypothalamus or posterior pituitary-areas that store and release ADH. Common causes include head trauma, surgical injury, tumors, and inflammatory diseases.
Patients typically notice a sudden increase in urination (polyuria) and an unquenchable thirst (polydipsia). Without treatment, dehydration and high blood sodium (hypernatremia) can develop quickly.
Understanding Pituitary Gland Dysfunction
Pituitary Gland Dysfunction covers any condition that impairs the gland’s ability to produce its suite of hormones, including growth hormone, ACTH, TSH, prolactin, and the ADH‑controlling posterior lobe. The gland sits at the base of the brain, acting as the master regulator for endocrine activity. When its cells are damaged-by a tumor, infarction, infection, or radiotherapy-hormone output can drop dramatically.
Because ADH originates in the hypothalamus and is stored in the posterior pituitary, any insult that reaches these regions can blunt ADH release, creating a direct pathway to CDI.
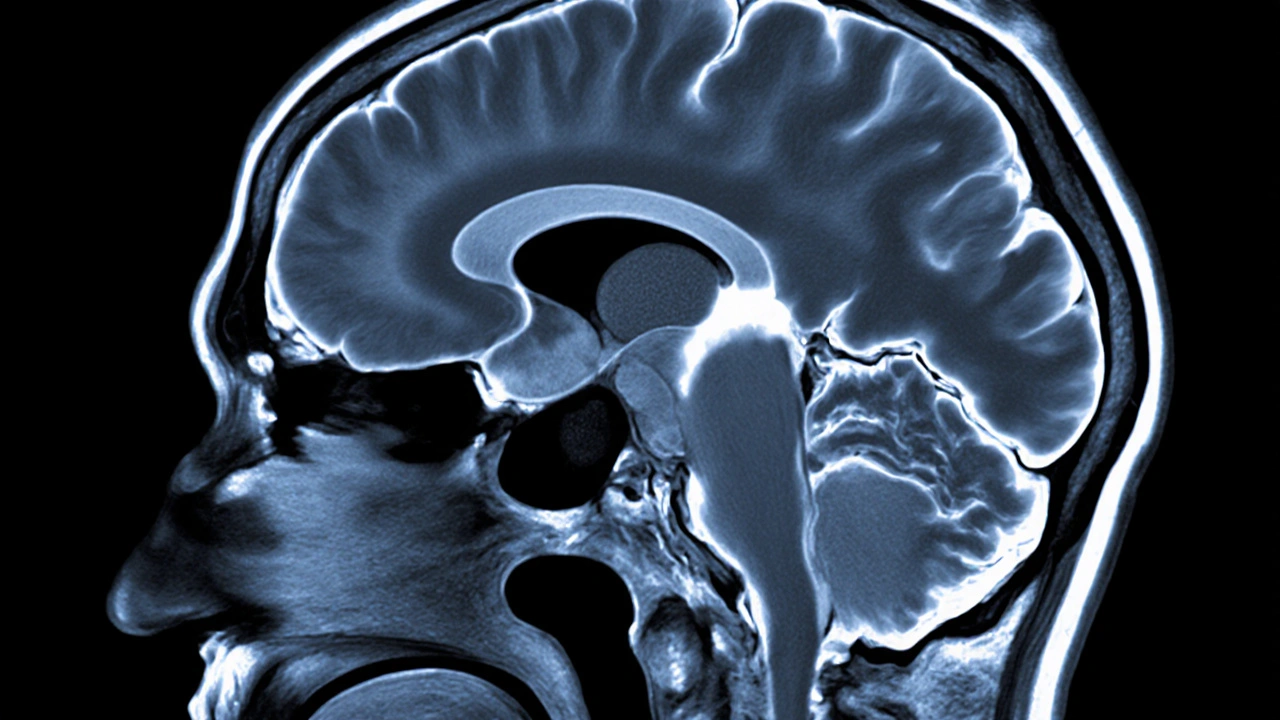
How the Hypothalamic‑Pituitary Axis Links the Two
The hypothalamus crafts ADH and sends it down nerve fibers to the posterior pituitary, where it’s released into the bloodstream. This short circuit is known as the hypothalamic‑pituitary‑ADH axis. Disruption at any point-hypothalamic injury, stalk compression, or posterior pituitary necrosis-cuts off the hormone flow.
Antidiuretic Hormone (ADH) binds to receptors in the kidney’s collecting ducts, prompting them to re‑absorb water back into the bloodstream. Without ADH, the kidneys let water pass freely, resulting in massive urine output. The link becomes evident when clinicians see a patient with a pituitary adenoma (a benign tumor) who also displays classic CDI symptoms.
Symptoms That Overlap and How to Spot Them
- Polyuria: often >3L per day, sometimes up to 20L in severe cases.
- Polydipsia: intense, constant thirst, especially for cold water.
- Nocturia: waking multiple times at night to urinate.
- Weight loss: due to calorie loss from constant urination.
- Hypernatremia: serum sodium >145mmol/L, indicating dehydration.
Because many pituitary disorders also cause hormonal imbalances (e.g., low cortisol leading to fatigue), the clinical picture can be muddled. A detailed history that notes the timing of symptom onset relative to any head injury, surgery, or diagnosed tumor helps narrow the cause.
Diagnostic Toolbox: Pinpointing the Cause
Accurate diagnosis hinges on three pillars: biochemical testing, functional testing, and imaging. Below is a quick comparison of the most frequently used tools.
| Test | Purpose | Key Findings for CDI |
|---|---|---|
| Water‑Deprivation Test | Assess kidney’s concentrating ability | Urine osmolality stays low despite dehydration; rises sharply after desmopressin. |
| Serum Sodium & Osmolality | Detect electrolyte imbalance | Elevated sodium (>145mmol/L) and high plasma osmolality. |
| MRI of Brain‑Pituitary Region | Visualize structural causes | Absent or thinning posterior pituitary bright spot; tumor, infarct, or stalk compression visible. |
| Vasopressin (Desmopressin) Response Test | Confirm ADH deficiency | Rapid increase in urine osmolality after synthetic ADH administration. |
When a patient shows the classic triad of polyuria, polydipsia, and hypernatremia, start with a basic serum sodium check. If the sodium is high, move quickly to a water‑deprivation test. MRI follows if a structural lesion is suspected.
Treatment Pathways: Managing Both Conditions Together
Therapy splits into two tracks: replacing the missing hormone and addressing the underlying pituitary problem.
Desmopressin is a synthetic analogue of ADH that can be given orally, nasally, or by injection to reduce urine output. Dosage is individualized; patients start low and titrate until urine volume falls below 2L per day and serum sodium normalizes.
If a pituitary adenoma is the culprit, neurosurgical resection or focused radiotherapy may restore normal hormone pathways. In cases where the damage is irreversible (e.g., post‑traumatic necrosis), lifelong desmopressin remains the mainstay.
Adjunctive care includes:
- Fluid management: Encourage balanced water intake to avoid both dehydration and dilutional hyponatremia.
- Electrolyte monitoring: Check sodium every 2‑4 weeks during dose adjustments.
- Hormone replacement: Evaluate for concurrent deficiencies (ACTH, TSH) that may need glucocorticoids or levothyroxine.
Practical Checklist for Clinicians and Patients
- Confirm polyuria (>3L/24h) and polydipsia through a symptom diary.
- Order serum sodium and osmolality; flag >145mmol/L.
- Schedule a water‑deprivation test if labs suggest ADH deficiency.
- Obtain a pituitary MRI to look for structural lesions.
- Initiate low‑dose desmopressin; adjust based on urine output and sodium.
- Screen for other pituitary hormone deficits.
- Arrange follow‑up imaging 6‑12months post‑intervention to assess lesion changes.
Patients should keep a daily log of fluid intake, urine volume, and any symptoms like headache or visual changes. This data helps the care team fine‑tune treatment and spot emerging complications early.
Frequently Asked Questions
Can central diabetes insipidus resolve on its own?
Rarely. Most cases stem from permanent damage to the hypothalamic‑pituitary axis, so hormone replacement is usually required for life. Occasionally, postoperative inflammation subsides and ADH secretion recovers, but this is the exception.
Is the MRI “bright spot” important?
Yes. On T1‑weighted images the posterior pituitary normally appears bright because of stored ADH. Its absence or reduction often signals central diabetes insipidus, especially when correlated with clinical findings.
What are the risks of desmopressin therapy?
If the dose is too high, patients can retain water, leading to hyponatremia and potentially seizures. Regular sodium checks and patient education on fluid balance mitigate this risk.
Do all pituitary tumors cause diabetes insipidus?
No. Only tumors that compress or destroy the posterior pituitary or its stalk typically impair ADH release. Macroadenomas affecting the anterior lobe may cause other hormonal imbalances without affecting water balance.
How fast can symptoms improve after starting desmopressin?
Most patients notice a reduction in urine volume within hours and a decrease in thirst over the first few days. Full normalization of serum sodium may take a week, depending on baseline severity.





12 Comments
Wow, this article really drags us through the hormonal maze!!! The pituitary drama is palpable, but the explanation feels half‑cooked, like a TV soap with too many plot twists!!!
Keep pushing forward-understanding CDI is the first step to mastering your health!
In many South Asian clinics we see the same confusion between pituitary tumors and water‑balance issues; sharing this guide will help bridge that gap for patients everywhere!
While your enthusiasm is noted, the article fails to cite recent peer‑reviewed studies that challenge the proposed diagnostic algorithm; a more rigorous citation list is indispensable.
You’ve got this-track your fluids, and the numbers will follow!
Imagine your kidneys as a thirsty desert oasis; desmopressin is the rain that finally quenches the longing, turning torrents of urine into a gentle stream!
Totally feel you, but let’s not forget that over‑hydrating can backfire; balance is the real hero here.
The endocrine system, with its intricate feedback loops, never ceases to amaze even the most seasoned clinicians.
When the hypothalamus decides to withhold antidiuretic hormone, the downstream effects ripple through the kidneys like a storm across a quiet lake.
Patients then experience polyuria, a symptom that, on the surface, appears simple but actually masks a cascade of osmotic imbalances.
Moreover, the thirst that follows is not merely a desire for water but a physiological alarm bell ringing at the cellular level.
Diagnostically, the water‑deprivation test remains the gold standard, yet it is often misinterpreted due to a lack of standardized protocols.
Serum sodium, while a useful marker, can be confounded by concurrent adrenal insufficiency, making clinical judgment paramount.
Imaging, particularly the high‑resolution MRI, offers a visual confirmation, but the notorious ‘bright spot’ can be absent in up to forty percent of confirmed cases.
Such variability demands that clinicians adopt a multimodal approach rather than relying on a single test.
Therapeutically, desmopressin is a marvel of synthetic hormone therapy, yet its dosing must be meticulously titrated to avoid hyponatremia.
The risk of water retention underscores the necessity for patient education, a point sometimes glossed over in hurried consultations.
Surgical intervention for pituitary adenomas can, in some instances, restore endogenous ADH production, but the outcomes are far from guaranteed.
Radiation therapy, while effective at tumor control, may further compromise posterior pituitary function, adding another layer of complexity.
Long‑term follow‑up should therefore include periodic assessment of both hormonal axes and renal function.
In practice, a collaborative care model involving endocrinologists, nephrologists, and neurosurgeons yields the best patient outcomes.
Ultimately, recognizing the tight coupling between pituitary health and water balance transforms a puzzling clinical picture into a manageable therapeutic journey.
Fantastic rundown! Let’s take this knowledge to the bedside and smash those diagnostic delays-action now!
America leads in endocrine research and knows the answer to CDI better than anyone.
Ah, the hubris of claiming solitary supremacy neglects the global tapestry of scientific inquiry, where every continent contributes a thread to the grand narrative of pituitary science.
I hear you, and it’s great that you’re tracking fluids; keep sharing your progress so the community can celebrate each milestone together.