When you take a medication, you might assume that a generic version is just as safe and effective as the brand-name one. For most drugs, that’s true. But for narrow therapeutic index drugs, the difference between a safe dose and a dangerous one is razor-thin. Getting the wrong version - or even a slight change in formulation - can mean the difference between recovery and hospitalization.
What Exactly Are Narrow Therapeutic Index Drugs?
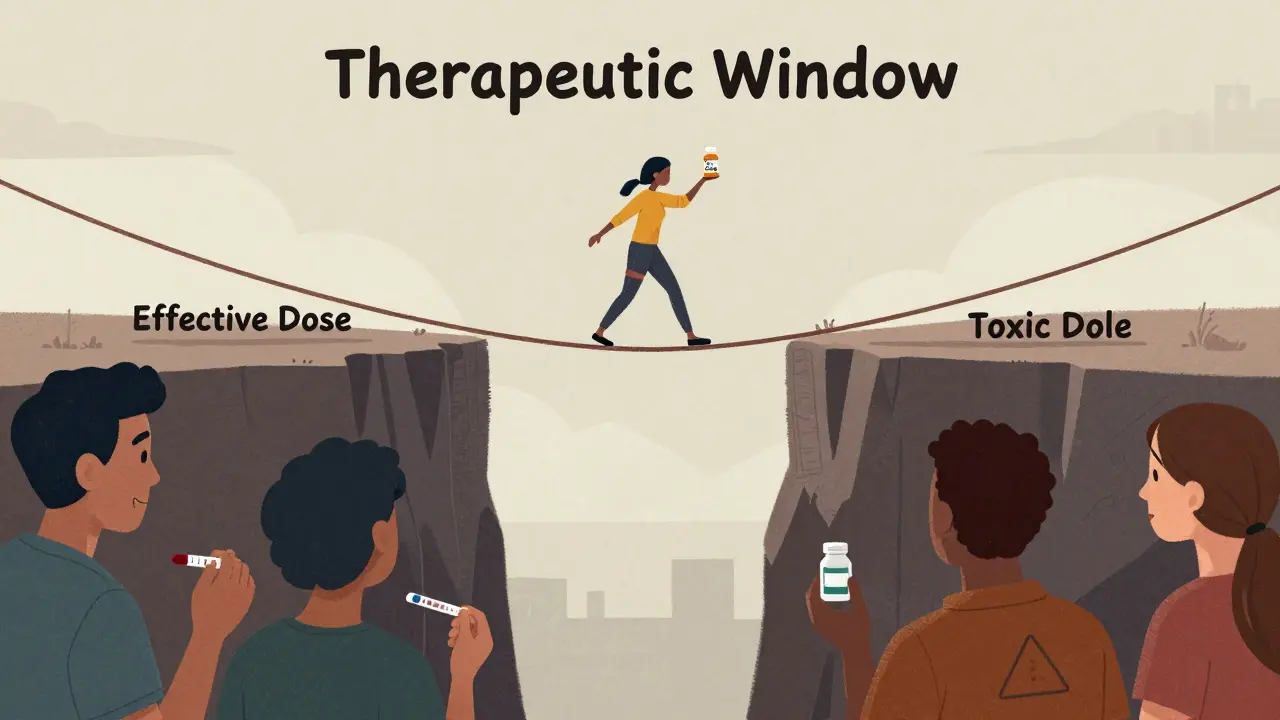
Narrow therapeutic index (NTI) drugs are medications where even tiny changes in blood levels can cause serious harm. The gap between the dose that works and the dose that poisons you is extremely small. The FDA defines them as drugs where small differences in dose or blood concentration can lead to life-threatening side effects or treatment failure. Think of it like walking a tightrope. Most drugs let you wobble a little. NTI drugs don’t. A 10% drop in blood level might mean the drug stops working. A 10% rise could trigger toxicity. That’s why these drugs are used only for conditions where precision is non-negotiable: preventing organ rejection after a transplant, controlling seizures in epilepsy, or keeping blood from clotting or breaking down too easily. Common NTI drugs include:- Warfarin (blood thinner)
- Tacrolimus and cyclosporine (immunosuppressants)
- Phenytoin and carbamazepine (anti-seizure meds)
- Levothyroxine (thyroid hormone)
- Digoxin (heart medication)
- Aminoglycoside antibiotics
- Newer cancer drugs like axitinib and nilotinib
Why Generic Versions of NTI Drugs Are a Big Deal
For most medications, generics are approved if they deliver the same amount of drug into the bloodstream as the brand-name version, within a range of 80% to 125%. That’s called bioequivalence. But for NTI drugs, that range is way too wide. The FDA requires much tighter standards. For many NTI drugs, the acceptable range for generics is narrowed to 90% to 111%. For drugs with very low variability in how patients absorb them, it can be as tight as 95% to 105%. That’s a huge difference. It means generic manufacturers have to prove their product behaves almost identically to the brand - not just in lab tests, but in real patients. This isn’t just bureaucracy. It’s life-or-death science. A 2023 study showed that patients on generic tacrolimus after a kidney transplant had a 30% higher chance of their creatinine levels spiking - a sign their body was rejecting the organ - compared to those who stayed on the brand. One patient on a transplant forum wrote: “After switching to generic Prograf, my creatinine doubled in three weeks. I ended up in the hospital.” But it’s not all bad news. Another patient with epilepsy reported five years on generic phenytoin with no seizures and $300 monthly savings. So why the difference? The problem isn’t generics themselves. It’s switching.The Real Issue: Switching Between Brands and Generics
Patients do fine on a single generic version - if they stay on it. The danger comes when they’re switched between different brands, or between brand and generic, or even between different generic manufacturers. Each manufacturer uses slightly different fillers, coatings, and manufacturing processes. For a drug like levothyroxine, those tiny differences can change how much hormone gets absorbed. One pharmacist on Reddit shared: “I’ve seen TSH levels swing wildly after switching generics. One patient went from 2.1 to 8.7 in two weeks. She was exhausted, gaining weight, depressed. It took three months to stabilize her.” This is why pharmacists are cautious. A national survey found only 28% of pharmacists would automatically substitute a generic for an NTI drug on a first prescription - compared to 78% for regular drugs. And 64% believe substitution could lead to therapeutic failure. The FDA recommends that prescribers write “dispense as written” on prescriptions for NTI drugs when switching could be risky. Many doctors now do this automatically for transplant patients, epilepsy patients, or those on warfarin.
Therapeutic Drug Monitoring: Your Safety Net
If you’re on an NTI drug, regular blood tests aren’t optional - they’re essential. This is called therapeutic drug monitoring (TDM). It’s how doctors make sure your blood level stays in the safe zone. For example:- Warfarin users get regular INR tests to check clotting time.
- Tacrolimus levels are checked weekly at first, then monthly.
- Phenytoin levels are monitored to avoid toxicity symptoms like tremors or slurred speech.
State Laws and Insurance Policies Can Hurt You
As of 2023, 42 states have laws that restrict automatic substitution of NTI drugs. But the rules vary wildly. Some states require the prescriber to specifically allow substitution. Others let pharmacists switch unless the doctor says no. A few have no rules at all. Insurance companies often push for generics to cut costs. But if your plan forces you to switch from a brand to a generic without your doctor’s approval, you could be at risk. Always check your prescription label. If it says “substitution permitted,” ask your pharmacist if it’s safe for your drug. The Joint Commission, which accredits hospitals, now requires that NTI drug levels be documented in patient records as part of medication safety standards. That means if you’re in the hospital, your levels should be tracked - but outside the hospital, it’s up to you and your doctor to stay on top of it.What You Can Do to Stay Safe
If you take an NTI drug, here’s what you need to do:- Know your drug. Is it on the FDA’s NTI list? Ask your pharmacist or check the FDA’s website.
- Stick with the same product. Once you find a brand or generic that works, don’t switch unless your doctor says so. Even switching between two different generics can cause problems.
- Get blood tests. Don’t skip your TDM appointments. If your doctor doesn’t schedule them, ask.
- Write “dispense as written” on your prescriptions. If you’ve had issues before, ask your doctor to add this note. It legally prevents substitution.
- Track your symptoms. Fatigue, dizziness, unusual bruising, seizures, or heart palpitations after a switch? Call your doctor immediately. Don’t wait.
The Future: Personalized Dosing and Genetic Testing
The field is moving toward precision medicine. Researchers are studying how genes affect how people process NTI drugs. For example, some people metabolize warfarin slowly because of a genetic variant. Giving them a standard dose can lead to dangerous bleeding. By 2028, GlobalData predicts 40% of NTI drug prescriptions will include genetic testing - up from just 15% in 2023. This could make dosing safer and reduce the need for constant blood tests. Meanwhile, the FDA is working on 12 new product-specific guidances for newer NTI cancer drugs by 2025. International agencies like the EMA and PMDA are still catching up - Japan doesn’t even have a formal definition for these drugs yet. That means global drug development is still messy.Bottom Line: Generics Are Fine - If You’re Careful
Generic NTI drugs aren’t inherently unsafe. Many patients use them successfully for years. But the risk comes from switching, inconsistent manufacturing, and lack of monitoring. The system isn’t perfect. But if you know your drug, stay consistent, demand blood tests, and speak up when something feels off - you can manage it safely. Don’t let cost savings override safety. Your life depends on that narrow window between effective and toxic.Are all generic drugs unsafe?
No. Most generic drugs are just as safe and effective as brand-name versions. The issue is specific to narrow therapeutic index (NTI) drugs, where tiny differences in absorption can cause serious harm. For drugs like antibiotics or blood pressure meds, generics are routinely switched without risk. But for drugs like warfarin, tacrolimus, or levothyroxine, switching can be dangerous.
Can I switch from brand to generic safely?
Only under close medical supervision. If you’re on an NTI drug, switching from brand to generic - or between generics - requires careful monitoring. Your doctor should order blood tests before and after the switch to confirm your levels are stable. Never switch on your own, even if your insurance forces it. Ask your doctor to write “dispense as written” on your prescription if you’ve had problems before.
Why do some people do fine on generics while others don’t?
It depends on the drug, the person, and consistency. Some patients metabolize drugs predictably and stay stable on any version. Others have sensitive systems or multiple health conditions that make them more vulnerable to small changes. The biggest factor is switching. Patients who stay on the same manufacturer’s product - whether brand or generic - rarely have issues. Problems arise when they’re moved between different versions.
What should I do if my pharmacy switches my NTI drug without telling me?
Call your doctor immediately. Then call your pharmacy and ask for the name of the manufacturer of the new pill. Check the label - it should list the manufacturer. If you notice new symptoms like fatigue, dizziness, irregular heartbeat, or seizures, get a blood test right away. You have the right to request the original product. If your insurance won’t cover it, ask your doctor to file a prior authorization or medical exception.
Is there a list of NTI drugs I can check?
Yes. The FDA publishes a list of drugs with product-specific guidances for NTI status. As of early 2024, this includes 33 drug products across 14 active ingredients like warfarin, tacrolimus, phenytoin, levothyroxine, and digoxin. You can find the list on the FDA’s website under “Generic Drugs: Bioequivalence.” Ask your pharmacist to confirm whether your drug is on it.









15 Comments
Man, this post hit me right in the feels. I’ve been on warfarin for six years now, and I swear by the same generic brand. Switched once out of curiosity-big mistake. My INR went from 2.4 to 4.8 in two weeks. Ended up in the ER with a nosebleed that wouldn’t stop. Now I keep the pill bottle with the manufacturer name taped to my fridge. Don’t let insurance push you around. Your life isn’t a cost center.
And yeah, the FDA’s tighter standards for NTI drugs? Good. But why do so many states still let pharmacists swap them without telling you? That’s a loophole that needs to get slammed shut. Doctors aren’t mind readers. If you’re on one of these drugs, you gotta be your own advocate. Write ‘dispense as written.’ Say it loud. Say it often.
I even started a little spreadsheet tracking my labs. Not because I’m weird, but because I’ve seen what happens when you assume everything’s fine. It’s not paranoia. It’s precision. And in this game, precision saves lives.
Ugh. Another one of these ‘generics are evil’ rants. You people are so scared of saving money. I’ve been on generic levothyroxine for 8 years. Never had an issue. Your fear is just capitalism’s guilt trip dressed up as medicine. Wake up. Big Pharma is the real villain here, not generics. Stop letting corporations make you paranoid about pills that cost 1/10th the price.
wait so u mean if i switch from one generic to another its like playing russian roulette?? i thought all generics were the same?? like how do they even make them different?? are they using different sugar?? or something?? this is wild. my doc just swaps me out like its a cereal box. i thought that was the whole point of generics??
Oh wow. So you’re telling me people actually *trust* their meds? How quaint. You know what’s really dangerous? Not knowing your own body. If you need blood tests every other week to make sure a pill isn’t killing you, maybe you shouldn’t be on it at all. Or maybe you’re just one of those people who can’t handle responsibility. I’ve been on three different generics for my seizure med and I’m fine. Guess I’m just more evolved than the rest of you.
Also, ‘dispense as written’? That’s just a fancy way of saying ‘I can’t handle change.’ Grow up.
Y’all need to stop panicking and start listening. I’m a nurse and I’ve seen this play out a hundred times. A sweet 72-year-old lady switches from brand to generic tacrolimus because her insurance says so. Two weeks later, she’s shaking, nauseous, and her kidney numbers are screaming. We had to admit her. She cried and said, ‘I didn’t know it was a big deal.’
It IS a big deal. And it’s not about being scared-it’s about being smart. If your drug is on the NTI list, treat it like your car’s brakes. You don’t swap brake pads from random online sellers. You go to the same place. You check the specs. You trust the process.
And if your pharmacist tries to swap it without asking? Say no. Loudly. Then call your doctor. Don’t wait. Your life isn’t a lab experiment. It’s yours. Protect it.
The notion that generic substitution for narrow therapeutic index drugs is somehow ‘safe’ under current regulatory frameworks is not only misleading-it is dangerously negligent. The FDA’s bioequivalence standards, even when tightened to 90–111%, still permit clinically significant variability in absorption kinetics across patient populations. This is particularly problematic in elderly patients with altered gastrointestinal motility or those on polypharmacy regimens.
Moreover, the lack of uniform state legislation creates a patchwork of risk. In states where substitution is permitted absent explicit prohibition, pharmacists-who are not trained to interpret therapeutic drug monitoring data-are effectively making clinical decisions without accountability. This is not pharmaceutical policy. This is institutionalized negligence dressed in bureaucratic language.
The solution is not merely patient education. It is systemic reform: mandatory electronic alerts in pharmacy systems, universal ‘dispense as written’ mandates for NTI drugs, and centralized national registries tracking substitution events and adverse outcomes. Until then, we are not managing medication safety-we are gambling with human lives.
USA is the only country that lets big pharma rip people off with brand name drugs. Other countries use generics just fine. You Americans are weak. You cry over a $5 pill. We don’t need your drama. My cousin in Germany takes generic warfarin. He’s fine. He doesn’t need a spreadsheet. He doesn’t need blood tests every week. He just takes his pill and lives. You people are too soft.
Stop whining. Get over it. Generic is cheaper. Generic is better. End of story.
Hold up. So you’re saying the FDA knows this is a problem but doesn’t fix it? And insurance companies are forcing switches? And states have no rules? And pharmacists don’t tell you? And doctors don’t always monitor?
Wait. This isn’t a medical issue. This is a conspiracy. Someone’s making money off your fear. Who’s behind this? Who owns the labs? Who owns the generic makers? Who owns the FDA? Why is no one asking these questions?
I’m not saying you’re wrong. I’m saying you’re being played. The real NTI drug isn’t warfarin. It’s the system.
Oh honey. You think this is about safety? Sweetie, it’s about power. The people who wrote the FDA guidelines? They used to work for the brand-name companies. The pharmacists who ‘recommend’ switching? They get kickbacks from the generics. The doctors who don’t order labs? They’re too busy playing golf to care.
You’re not being cautious. You’re being manipulated. You think your spreadsheet is saving you? It’s just a prop in the performance. The real danger isn’t the pill. It’s the illusion that anyone in this system actually gives a damn about you.
Go ahead. Take your blood tests. Write ‘dispense as written.’ Keep your pill bottle labeled. But don’t pretend you’re in control. You’re not. You’re just the audience for a very expensive tragedy.
so like if i switch from one generic to another and my head starts spinning is that my fault or the pill's fault?? i dont get it. my doc just says 'take it' and i do. why is this so complicated. also why do they even make different versions if they're supposed to be the same??
The entire discourse around NTI drugs reveals a fundamental epistemological flaw in contemporary pharmacology: the conflation of bioequivalence with therapeutic equivalence. The FDA’s 90–111% range is not a scientific threshold-it is a regulatory compromise born of economic expediency.
And yet, patients are expected to internalize this as ‘safe.’ This is not medicine. It is neoliberal biopolitics in action: reducing human physiology to a cost-benefit algorithm while outsourcing responsibility to the individual. The ‘dispense as written’ directive is not empowerment-it is a Band-Aid on a hemorrhage.
Meanwhile, genetic testing for CYP450 variants remains inaccessible to 87% of the U.S. population due to insurance denials. The future of precision medicine is here. But only for those who can afford it.
i just want to say thanks for writing this. i’ve been on cyclosporine since my transplant and i never knew any of this. i thought all pills were the same. i’ve been switching generics because my insurance said so. i just found out last week that my levels were off. i didn’t even realize i was having side effects until i read this.
now i’m calling my doc tomorrow to lock in my brand. i’m not taking any chances. you’re right-this isn’t about money. it’s about staying alive. i’m gonna start tracking my labs too. i don’t care if it’s weird. i’d rather be annoying than dead.
thanks for not making me feel dumb for not knowing this.
Let me guess-you’re one of those people who thinks the government is watching your meds. Newsflash: the FDA doesn’t care about you. They care about profits. And the ‘tightened’ standards? That’s just PR. The same companies that make the brand drugs make the generics. Same factories. Same people. The only difference? The label.
And don’t get me started on ‘dispense as written.’ That’s not patient safety. That’s corporate lobbying. Big Pharma doesn’t want you switching because they’re scared you’ll realize their $500 pill is the same as the $5 one.
Wake up. This isn’t science. It’s a scam. And you’re falling for it.
bro i switched from brand to generic phenytoin last year and my seizures got worse. i thought it was stress. turns out my blood level dropped 40%. i was like 80% of the way to toxic. i almost died. now i only take the brand. my insurance hates me. i don’t care. i’d rather pay $400 a month than end up in a hospital with my brain on fire.
also-why do people act like this is new? i’ve been screaming about this since 2017. nobody listened. now you’re all like ‘oh wow this is wild.’ yeah. it’s been wild for years. you just didn’t care until it happened to you.
Thank you for this comprehensive and clinically accurate exposition. The nuances of narrow therapeutic index pharmacokinetics are too frequently oversimplified in public discourse. The distinction between bioequivalence and therapeutic equivalence remains critically underappreciated by both patients and prescribers.
I would further emphasize that therapeutic drug monitoring should not be viewed as an optional adjunct-it is the standard of care. The American Society of Health-System Pharmacists’ recommendation for 16 hours of continuing education for pharmacists managing NTI drugs is a minimum, not a target.
It is imperative that healthcare systems institutionalize protocols for NTI drug management, including mandatory electronic alerts, pharmacist-led TDM interpretation, and patient education pathways. The current reliance on individual vigilance is unsustainable and ethically inadequate.