Switching from a brand-name pill to a generic version should be simple - same active ingredient, same effect, cheaper price. But for some people, it’s not that easy. You might start taking the generic version of your blood pressure med, antidepressant, or thyroid pill and suddenly feel off. Nausea. Headaches. Trouble sleeping. Or worse - your symptoms come back. It’s not all in your head. The problem might not be the medicine you need - it’s what’s holding it together.
What Are Inactive Ingredients, Really?
The active ingredient in your pill is what actually treats your condition. But that’s only a small part of what’s inside. The rest? That’s the inactive ingredients - also called excipients. These aren’t meant to cure anything. They’re the support crew: the glue, the filler, the coating, the flavor. Think of them like the frame, paint, and tires on a car. The engine (active ingredient) does the work, but without the rest, it wouldn’t run smoothly - or even at all.
Common inactive ingredients include:
- Fillers: Lactose, microcrystalline cellulose, starch - they give tablets their size and shape.
- Binders: Povidone, hydroxypropyl methylcellulose - hold everything together so the pill doesn’t crumble.
- Disintegrants: Croscarmellose sodium, sodium starch glycolate - help the pill break apart in your stomach so the medicine can be absorbed.
- Coatings: Hydroxypropyl methylcellulose, polyethylene glycol - make pills easier to swallow or control how fast the drug releases.
- Preservatives and dyes: Sodium benzoate, FD&C dyes, parabens - keep the medicine from spoiling or make it look different.
These ingredients are approved by the FDA as safe for most people. But "safe for most" doesn’t mean safe for everyone.
Why Do Generics Feel Different?
By law, a generic drug must contain the same active ingredient in the same amount as the brand-name version. The FDA requires it to be bioequivalent - meaning your body absorbs it at nearly the same rate and level. That’s why 95% of people won’t notice a difference.
But here’s the catch: the FDA doesn’t require generics to match the brand’s inactive ingredients. So while your generic lisinopril has the same blood-pressure-lowering chemical as the brand, it might use corn starch instead of lactose. Or a different dye. Or a different coating that dissolves slower.
That’s enough to change how the medicine behaves in your body - especially if you’re sensitive.
For example:
- If you’re lactose intolerant (about 1 in 3 Americans are), a generic version with lactose as a filler could cause bloating, gas, or diarrhea.
- If you’re allergic to certain dyes like FD&C Red No. 40, you might get a rash or hives from a differently colored pill.
- If your thyroid medication uses a slower-dissolving coating, your body might not absorb enough of the hormone - and your TSH levels could spike.
These aren’t myths. In a 2021 study of 2,000 patients switching from brand to generic, 4.3% reported side effects linked to inactive ingredients. About 1% had to switch back because the difference was too noticeable.
Who’s Most at Risk?
Most people can switch without issue. But some groups need to be extra careful:
- People with narrow therapeutic index drugs: These are medications where the difference between a helpful dose and a harmful one is tiny. Examples: levothyroxine (Synthroid), warfarin (Coumadin), digoxin, phenytoin, and lithium. Even small changes in absorption can throw your levels out of balance.
- People with food or chemical sensitivities: Lactose, gluten, sulfites, soy, or artificial dyes can trigger reactions - even in tiny amounts found in pills.
- People on multiple medications: If you’re taking five or more pills, you’re more likely to have overlapping inactive ingredients that build up and cause side effects.
- Older adults: Slower metabolism and changes in stomach acid can make absorption less predictable, making formulation differences more noticeable.
One patient I spoke with switched from brand-name Synthroid to a generic and felt fine at first. But after three weeks, her fatigue came back. Her TSH jumped from 2.1 to 7.8 - well outside the target range. She switched back. Her levels normalized in two weeks. Her doctor didn’t think it was possible - until he checked the inactive ingredients. The generic used a different coating that slowed absorption.

How to Spot the Difference
You don’t need a chemistry degree to protect yourself. Here’s what to do:
- Check the label. Look at the "Inactive Ingredients" section on the bottle or package insert. You can also search the FDA’s Inactive Ingredient Database (available online). If you see something like "lactose monohydrate" or "FD&C Blue No. 1" and you know you react to it - speak up.
- Ask your pharmacist. Pharmacists can tell you if your new generic has different fillers or coatings than your old one. They can also check if there’s another generic version with a different formulation.
- Keep a journal. For two to four weeks after switching, write down how you feel: sleep, energy, mood, digestion, headaches. Note the date you switched and the name of the generic (it’s often printed on the pill).
- Don’t assume all generics are the same. There are often 5-10 different generic versions of one drug. One might use lactose, another might use rice starch. If one generic gives you trouble, try another.
Some pharmacies will even let you request a specific generic manufacturer - especially if you’ve had a bad experience before.
When to Call Your Doctor
Not every change means you need to switch back. But if you notice any of these after switching to a generic:
- Worsening of your original symptoms
- New side effects that weren’t there before
- Changes in lab results (like TSH, INR, or blood sugar levels)
- Allergic reactions (rash, swelling, trouble breathing)
Call your doctor. Don’t just stop the medicine. Your doctor can order tests, check your levels, or request a brand-name or authorized generic.
Authorized generics? They’re the same as the brand-name drug - same active ingredient, same inactive ingredients - just sold under a generic label at a lower price. They’re not always available, but they’re a great option if you’re sensitive.
Why Generics Still Matter
Let’s be clear: generics are safe, effective, and save lives. In the U.S., 9 out of 10 prescriptions are filled with generics. They’ve saved the healthcare system over $2 trillion in the last decade. For most people, switching saves hundreds - sometimes thousands - a year.
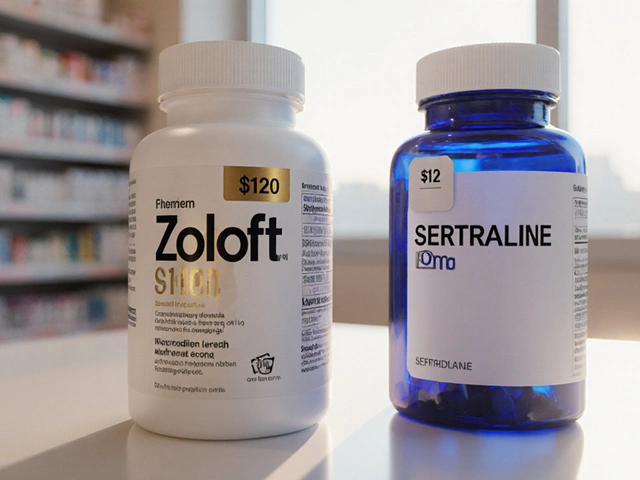
Take atorvastatin (Lipitor). The brand costs around $370 a month. The generic? About $4. That’s not a minor difference. It’s the difference between taking your medicine and skipping it because you can’t afford it.
The goal isn’t to scare you away from generics. It’s to help you use them wisely.
What’s Changing?
The FDA is paying more attention. In 2023, they released new draft guidance requiring stricter testing for generics of narrow therapeutic index drugs like levothyroxine. They’re also expanding their Inactive Ingredient Database, which will be fully public by early 2024. And by 2025, most major generic manufacturers plan to offer "allergen-free" versions of common medications - no lactose, no dyes, no sulfites.
Some companies are even moving toward "clean label" pills - using only ingredients you’d recognize from your kitchen. That’s a big shift.
But until then, you’re your own best advocate.
Final Advice: Don’t Guess. Check.
If you’ve ever felt "off" after switching to a generic - you’re not alone. And you’re not crazy. It’s not always the drug. It’s the delivery system.
Don’t suffer in silence. Don’t assume all generics are identical. Don’t let cost be the only factor if your body is telling you something’s wrong.
Check the label. Talk to your pharmacist. Track your symptoms. And if something doesn’t feel right - speak up. Your health isn’t a trade-off between price and comfort. It’s about finding the version that works for you.
Generics aren’t the enemy. But knowing what’s inside them? That’s power.





10 Comments
Generic pills are fine for most people but I switched to one and got a rash. Turned out it had red dye. I checked the label and switched back. Simple fix.
You people act like this is some big conspiracy. The FDA approves these things. If you’re sensitive to lactose or dyes, that’s your problem not the system’s. Stop whining and read the label like everyone else.
I’ve been on levothyroxine for 12 years. Switched generics three times. One made me feel like I was underwater. Another gave me heart palpitations. I finally found one that uses rice starch and no dyes. It’s like breathing again. Never assume they’re all the same. Keep a journal. Talk to your pharmacist. Your body knows.
The philosophical underpinnings of pharmaceutical equivalence are predicated upon a reductionist model of human physiology that fails to account for individual variability in pharmacokinetic absorption. The regulatory framework, while statistically sound, is epistemologically inadequate when applied to the phenomenological experience of the patient. Hence, the assertion that generics are interchangeable constitutes a category error in clinical practice.
I switched to a generic and felt like I was dying for two weeks. My anxiety went through the roof. I cried every night. My partner didn’t believe me. My doctor said it was all in my head. Then I checked the label and saw FD&C Yellow No. 6. I had no idea I was allergic. I’m so mad I almost didn’t tell anyone because I thought I was crazy. But I’m telling you now. You’re not crazy. It’s real.
How quaint. You Americans treat medication like a luxury good. In India, we take whatever is available. If you’re sensitive to fillers, perhaps your body is too fragile for modern medicine. The real issue is not the pill-it’s your entitlement to perfection.
My mom takes warfarin and switched generics last year. Her INR went wild. She didn’t say anything for weeks because she didn’t want to bother anyone. Finally we checked the bottle and the new one had a different binder. Pharmacist switched her back to the old generic. She’s fine now. Just check the label. Don’t wait until you’re in the ER.
Listen, I’ve been a pharmacist for 20 years. I’ve seen people panic over generics. Most of the time it’s placebo. But not always. I’ve had patients come in with tears saying their thyroid med doesn’t work anymore. We check the manufacturer. Sometimes it’s the coating. Sometimes it’s the dye. I tell them: don’t suffer. Don’t guess. Ask for the exact version you used before. Or ask for an authorized generic. You deserve to feel well. It’s not too much to ask.
Everyone’s so sensitive these days. Lactose? Dyes? You think your body is special? I’ve taken generics my whole life. Never had an issue. If you can’t handle a little corn starch, maybe you shouldn’t be taking medicine at all. This is why healthcare costs are insane. People want branded pills for $4.
Just found out my antidepressant generic had soy lecithin. I’m allergic. Had a minor reaction. Now I screenshot the inactive ingredients every time I refill. 🛑🩺💡