For decades, generic drug makers followed a simple rule: copy the brand-name pill, test the final product, and hope it passes. If the tablet dissolved too slowly or had a slightly different impurity profile, the whole batch got rejected. It was like baking cookies by guessing the oven temperature-no real understanding of why they burned or stayed raw. That changed in 2009, when the Quality by Design (QbD) framework became the new standard for generic drug development. Today, it’s not enough to match the brand drug’s results-you have to prove you understand how and why it works.
What Is Quality by Design, Really?
QbD isn’t just another checklist. It’s a mindset. The International Council for Harmonisation (ICH) defines it as building quality into the product from the start, not testing it in at the end. That means asking: What does the drug need to do? What can go wrong during manufacturing? And how do we control it?
The foundation is the Quality Target Product Profile, or QTPP. This isn’t a vague wish list. It’s a precise blueprint: dissolution rate, impurity limits, tablet hardness, even how the drug breaks down in the body. For a generic to be approved, it must match the brand-name drug (called the Reference Listed Drug or RLD) in at least 95% of key performance metrics. No guesswork. No assumptions.
From Copycat to Scientist
Before QbD, developers picked a mixing time, a compression force, a drying temperature-and stuck to it. Change one number? You needed regulatory approval. That slowed things down and made scaling up risky.
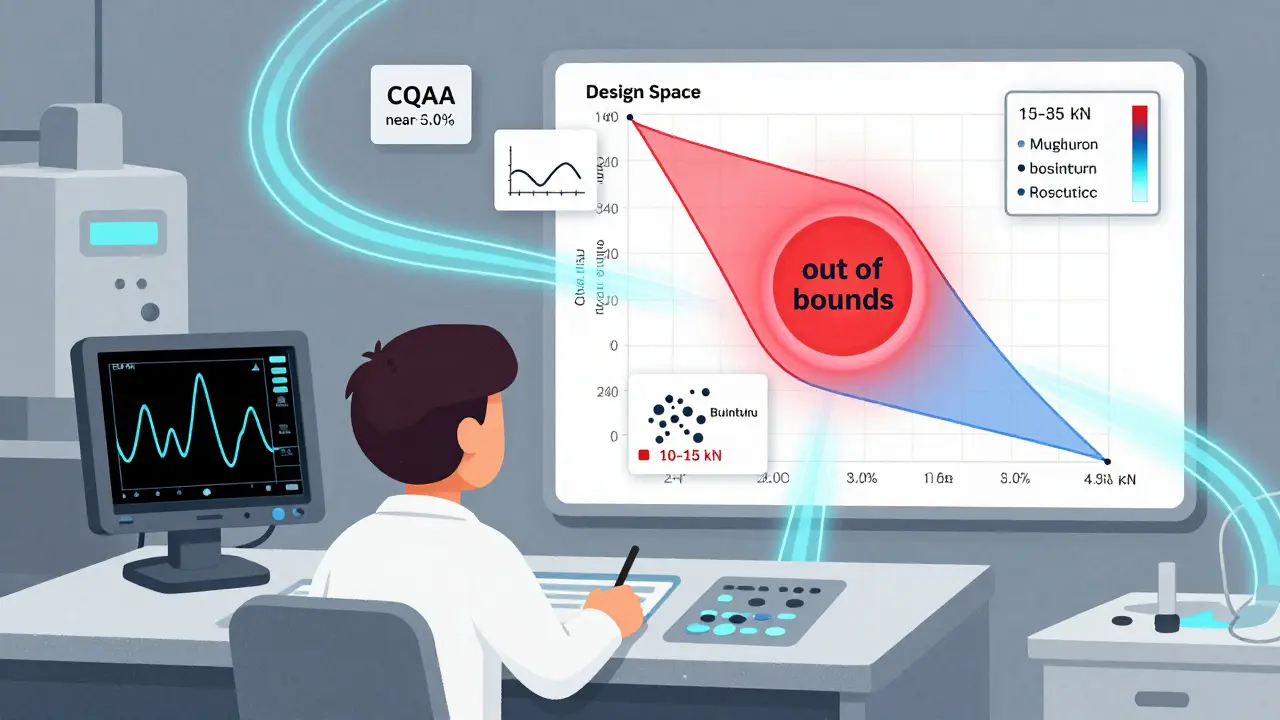
Now, QbD uses Design of Experiments (DoE) to map out how changes in process parameters affect the final product. Instead of one fixed setting, you define a range. For example, granulation moisture might be allowed between 1.5% and 3.0%. Compression force could swing from 10 to 15 kN. As long as you stay within these boundaries, you’re good. No extra paperwork. No delays.
This range is called the Design Space. The FDA accepts it if data shows 95% confidence that every batch made inside this space will meet all quality standards. That’s not luck. It’s science. And it’s why QbD-based applications now have a 92% first-cycle approval rate, compared to 78% for traditional ones.
What Makes a Quality Attribute Critical?
Not every feature of a drug matters equally. QbD forces you to pick the ones that actually impact safety or effectiveness. These are called Critical Quality Attributes, or CQAs.
For most oral generics, you’ll see five to twelve CQAs:
- Dissolution profile: Must match the RLD with an f2 similarity factor above 50
- Content uniformity: Each tablet must contain the right dose, with relative standard deviation under 6.0%
- Impurity levels: Must stay below ICH Q3B thresholds
- Particle size distribution: Especially important for poorly soluble drugs
- Tablet hardness and disintegration time
These aren’t arbitrary numbers. They’re tied to real-world performance. If dissolution is off by 10%, the drug might not absorb properly in the gut. That’s not just a failed test-it’s a patient who doesn’t get the medicine they need.
How Do You Prove It Works?
QbD doesn’t rely on end-product testing alone. It uses Process Analytical Technology (PAT) to monitor the process in real time. Think of it like a car’s dashboard-instead of waiting for the engine to break down, you watch the oil pressure, temperature, and RPM as you drive.
Tools like near-infrared spectroscopy let manufacturers check moisture content, blend uniformity, or coating thickness during production. That cuts end-product testing by 35-60%. One manufacturer cut their lab workload from 200 tests per batch to just 40.
This isn’t science fiction. In 2022, 87% of companies using QbD had PAT systems in place. And it’s paying off: Teva’s levothyroxine line, using continuous manufacturing with QbD, improved batch consistency by 28%.
Why the FDA Loves It
The U.S. Food and Drug Administration doesn’t just tolerate QbD-it requires it. Since October 2017, every Abbreviated New Drug Application (ANDA) must include QbD elements. Why? Because it works.
QbD submissions get approved 23% more often. Review times dropped by 4.7 months on average. Fewer requests for more data. Fewer Complete Response Letters (CRLs)-the dreaded "we need more info" notices that delay launches.
And when changes happen? Companies with approved Design Spaces can adjust parameters without waiting for approval. Mylan (now Viatris) made 11 process changes to their simvastatin production during the pandemic without a single regulatory hold. That meant 99.8% on-time delivery when supply chains were falling apart.
The Hidden Costs
QbD isn’t free. It’s expensive. Upfront costs can be 25-40% higher than traditional development. You need:
- Specialized training (80-120 hours per scientist on risk management and DoE)
- PAT equipment (minimum $500,000 investment)
- Statistical software like MODDE Pro ($15,000 per user per year)
- More time-adding 4 to 8 months to a typical 18-24 month development cycle
For a simple immediate-release tablet, this can feel like overkill. Dr. James Polli from the University of Maryland warned that some companies spent $450,000 on DoE studies for drugs where the design space was already well known. That’s not innovation-that’s waste.
That’s why smart companies use risk-based approaches. For multi-strength products, they test the highest and lowest doses and bracket the middle ones. That cuts studies by 45%. For low-cost generics, QbD should be proportionate-not perfect, just sufficient.
Where QbD Shines (and Where It Struggles)
QbD isn’t one-size-fits-all. It’s a superpower for complex products:
- Inhalers: Hard to match particle size and delivery without QbD
- Transdermal patches: Skin absorption varies wildly without controlled release profiles
- Modified-release tablets: Delayed or extended release needs precise layering and dissolution control
For these, traditional bioequivalence studies often fail. The body doesn’t respond the same way if the tablet breaks down differently-even if the final drug concentration looks identical.
But for simple immediate-release pills? QbD’s value is smaller. The FDA’s 2023 guidance says: Don’t over-engineer. Use existing knowledge. Leverage published RLD data. That’s what top Indian generics companies are doing-they’re investing in QbD, but focusing it where it matters most.
The Global Shift
QbD isn’t just a U.S. trend. The European Medicines Agency (EMA), Japan’s PMDA, and now the World Health Organization (WHO) all require it for complex generics. In 2022, 74% of new ANDAs included QbD elements-up from 38% in 2018. For complex products, adoption hits 92%.
The market for QbD consulting, software, and training is now $1.4 billion a year. PAREXEL and other firms help generic companies navigate the complexity. But the real winners are the manufacturers who mastered it.
Hikma Pharmaceuticals cut post-approval deviations from 14 per year to just 2 after switching to QbD. That saved $850,000 annually in recalls, investigations, and retests.
What’s Next?
The next wave is integration with continuous manufacturing and 3D printing. The FDA’s Emerging Technology Program has approved 27 QbD-based continuous manufacturing applications-with a 100% success rate.
By 2027, McKinsey predicts 95% of new generic approvals will use QbD. The tools are getting better. The data is getting richer. The regulatory bar is rising.
But the goal hasn’t changed: deliver safe, effective, consistent medicine-every time, no matter where it’s made. QbD doesn’t just meet that goal. It makes it predictable.
How to Start With QbD
If you’re new to QbD, here’s a practical roadmap:
- Define your QTPP based on the RLD’s known performance
- Identify your top 5-7 CQAs-focus on what impacts safety or efficacy
- Use risk assessment (ICH Q9) to find which process steps matter most
- Run a small DoE study on 3-5 critical parameters
- Build your Design Space using statistical confidence, not guesswork
- Integrate PAT tools where they add real-time control, not just data
- Document everything-not for regulators, but for your team
You don’t need to do everything at once. Start with one product. Master it. Then scale.
Common Mistakes to Avoid
- Trying to replicate the RLD exactly instead of understanding its performance
- Using DoE for simple products where the design space is already known
- Ignoring in vitro-in vivo correlation (IVIVC)-if your dissolution test doesn’t predict how the body absorbs the drug, it’s just a number
- Over-investing in equipment without training staff to use it
- Forgetting that QbD is a system, not a checklist
QbD isn’t about perfection. It’s about control. And control means fewer surprises, faster approvals, and better medicines for patients.
Is Quality by Design mandatory for all generic drugs?
Yes, since October 1, 2017, the U.S. FDA requires QbD elements in all Abbreviated New Drug Applications (ANDAs). The European Medicines Agency and Japan’s PMDA have similar expectations, especially for complex generics like inhalers or modified-release tablets. Even if not legally required for simple products, regulatory agencies now expect a science-based approach, making QbD the de facto standard.
How does QbD improve bioequivalence testing?
QbD doesn’t replace bioequivalence studies-it strengthens them. Instead of relying solely on clinical trials to prove a generic works like the brand, QbD uses detailed in vitro testing (like dissolution profiles) to predict in vivo performance. By establishing a Design Space that ensures consistent drug release, manufacturers can demonstrate bioequivalence without always needing human trials. This is especially critical for drugs with narrow therapeutic windows or complex delivery systems.
What’s the difference between a Critical Quality Attribute (CQA) and a Critical Process Parameter (CPP)?
A Critical Quality Attribute (CQA) is a property of the final drug product that affects safety or effectiveness-like dissolution rate or impurity levels. A Critical Process Parameter (CPP) is a variable in the manufacturing process that impacts a CQA-like granulation moisture, compression force, or drying temperature. You control CPPs to ensure your CQAs stay within acceptable limits.
Can small generic manufacturers afford QbD?
Yes, but strategically. You don’t need to do full DoE on every product. Use risk-based bracketing-test only the highest and lowest strengths of a multi-strength line. Leverage public RLD data to reduce testing. Start with one product. Use FDA’s free QbD training modules. Partner with consultants for complex cases. The goal isn’t to spend more-it’s to spend smarter. Companies that do this see ROI in fewer delays, lower rejection rates, and faster market access.
Does QbD reduce the need for clinical trials in generics?
For most traditional generics, clinical trials aren’t required under the ANDA pathway regardless of QbD. But QbD helps ensure that the in vitro data (like dissolution profiles) reliably predicts how the drug will behave in the body. For complex generics-where traditional methods fail-QbD can eliminate the need for costly and time-consuming clinical bioequivalence studies by proving equivalence through advanced analytical methods and robust process control.
What role does Process Analytical Technology (PAT) play in QbD?
PAT enables real-time monitoring of critical process parameters during manufacturing. Tools like near-infrared spectroscopy can check blend uniformity, moisture content, or coating thickness as the product is made. This replaces or reduces the need for end-product testing, catches deviations early, and ensures consistency across batches. Companies using PAT report 35-60% fewer lab tests and faster release times.
How long does it take to implement QbD in a generic drug project?
For a simple immediate-release tablet, expect 6-9 months to build the QbD framework. For complex products like extended-release tablets or inhalers, it’s 12-18 months. This includes defining the QTPP, identifying CQAs, running DoE studies, validating PAT tools, and building the control strategy. While it adds time upfront, it shortens regulatory review by 4-5 months and prevents costly delays later.






12 Comments
QbD isn't magic, it's just good science. Stop treating it like a burden and start seeing it as a tool.
Finally, someone who gets it.
Let me tell you something about QbD that no one else will admit-most of these so-called 'design spaces' are just statistical smoke and mirrors. Companies spend half a million dollars on DoE studies for a simple tablet because they think it makes them look sophisticated, not because it actually improves anything. The FDA loves it because it gives them more paperwork to review, not because it's always necessary. And don't even get me started on PAT equipment-$500k for a spectrometer that just tells you what you already knew from experience. This isn't innovation, it's regulatory theater dressed up in lab coats.
I appreciate how this breaks down the real value of QbD without hype. It's not about perfection-it's about predictability. That’s huge for patients who rely on consistent meds.
I keep thinking about how QbD shifts the focus from compliance to understanding. We used to test pills to see if they worked. Now we ask why they work. That’s a quiet revolution in medicine. It’s not just about data-it’s about respect for the science and the people who take the drugs.
You think this is about quality? Nah. This is Big Pharma’s way of squeezing out small generic makers. They know most Indian and Eastern European firms can’t afford $500k PAT systems. So they push QbD as a 'standard'-but it’s really a barrier to entry. And now the FDA enforces it like gospel. Who benefits? The same companies that made the brand-name drugs in the first place. It’s not science-it’s capitalism in a lab coat.
Thank you for this comprehensive overview 🙏
QbD is indeed a paradigm shift, and while the upfront cost is significant, the long-term benefits in terms of regulatory efficiency and product consistency are undeniable.
For developing nations, strategic adoption-not blanket implementation-is key. We must balance innovation with accessibility.
The distinction between CQAs and CPPs is non-negotiable in regulatory submissions. Failure to properly define and correlate these parameters constitutes a major deficiency under ICH Q11. Many applicants still conflate process control with end-product testing, which is fundamentally incompatible with QbD principles. If you’re not using risk-based approaches like ICH Q9 to prioritize your CQAs, you’re not doing QbD-you’re doing checkbox compliance.
OMG this is the most exciting thing to happen to generics since… ever?!
Finally, we’re treating medicine like engineering instead of alchemy. I cried when I read about Teva’s 28% consistency boost. That’s not just numbers-that’s real people getting reliable meds, every single time. And PAT? It’s like giving factories X-ray vision. I’m so here for this.
Let’s get more women and non-binary scientists in this space-this needs diverse minds!
I just saw a video of a lab tech crying because their $2M PAT system broke down and they had to do manual tests again. This whole QbD thing? It’s a trap. You think you’re being cutting-edge, but you’re just building a house of cards made of software licenses and consultant fees. And when it crashes? You’re stuck. Meanwhile, the guy in India who just eyeballs his tablet hardness? He’s still shipping 10 million pills a month without a single recall. Maybe we’re overcomplicating life.
In India, we started with one product-simple immediate-release. Used public RLD data, did minimal DoE, focused only on dissolution and uniformity. Saved 60% on costs. Got approved in 9 months. QbD doesn’t mean spending more. It means spending wisely.
I’ve seen too many teams treat QbD like a checklist instead of a mindset. The real win isn’t in the reports-it’s in the culture. When your chemist starts asking 'what if?' instead of 'what’s the rule?' that’s when change happens. Slow down. Listen. Let the science lead.
QbD is the future, no doubt. But let’s be real-most patients don’t care how the pill was made, as long as it works. In places like rural India, we need affordable, simple, reliable generics. QbD helps when it’s needed, but don’t turn every tablet into a PhD thesis. Sometimes, good enough is enough.